
台灣超音波檢查AI即時偵測辨識肝腫瘤運用獲獎
返回上一層台灣之光再添一樁!由好心肝基金會、臺大醫院、聰泰科技開發公司三方所共同研發「深度學習在超音波檢查肝腫瘤自動偵測與辨識之應用」軟體,昨(30日)在日本京都舉辦的第33屆亞太肝臟研究醫學會(the Asian Pacific Association for the Study of the Liver, APASL)」中榮獲「研究者獎(Investigator Award)」大獎,這是國內第一個完全由社會愛心挹注所完成的即時超音波檢查影像AI裝置,聰泰科技公司大力支持公益協助開發AI軟體,由臺大醫院影像醫學部主治醫師吳志宏醫學博士代表報告與受獎,吸引全球數千位肝臟醫師專家學者與會見證。
「人工智慧應用於肝腫瘤超音波影像之即時自動偵測及辨識裝置」的研發,當初由好心肝基金會執行長暨臺大家庭醫學部兼任主治醫師粘曉菁醫學博士主導,希望將台灣首位以超音波檢查診斷肝腫瘤權威、臺大醫院名譽教授暨好心肝基金會董事長許金川教授的數十年超音波判讀肝腫瘤珍貴經驗雲端化,讓醫療人員幫病人做腹部超音波檢查的同時,猶如身旁有肝腫瘤診斷經驗豐富的教授,輔助即時判定肝腫瘤的良惡性。
此研發搜集了2002年至2020年期間臺大醫院1576名患者共6001個肝臟腫瘤超音波影像,且透過電腦斷層(CT)或磁振造影(MRI)影像或病理結果確定肝腫瘤診斷,而後影像再由經過認證的超音波專科醫師標記出每一個腫瘤部位,進一步訓練AI軟體自動偵測及辨識肝腫瘤影像,並於超音波檢查同時,即時提供肝腫瘤良惡性判讀結果,影像良惡性辨識度高達9成,精確度比擬CT與MRI的診斷,此AI裝置可協助醫療人員以無輻射且快速的超音波檢查,即時輔助醫師診斷病友肝腫瘤的良惡性,緩解病人被診斷有肝腫瘤的焦慮感,還能減少高階影像(CT或MRI)檢查的等候時間與國家醫療資源的支出。
此肝腫瘤智慧分析裝置目前已取得多國專利證書,包括:中華民國、日本、歐盟、美國、中國大陸等,期盼不久的將來,AI裝置可以實際運用於臨床上,醫療人員使用無輻射又快速的超音波在幫民眾做檢查的同時,可輔助肝腫瘤早期診斷的精準度,緩解病人被診斷罹患肝腫瘤等候確診肝腫瘤良惡性的焦慮感,並可望減少高階影像檢查(CT或MRI)的不便性與國家醫療資源支出。

►國內第一個完全由社會愛心挹注所完成的即時超音波檢查影像AI裝置。

►台大醫院名譽教授暨好心肝基金會董事長許金川的數十年來與團隊合作,將無數超音波判讀肝腫瘤資料雲端化,如今培訓AI即時超音波檢查影像AI裝置輔助變身名醫的重要素材。

►好心肝基金會執行長暨臺大家庭醫學部兼任主治醫師粘曉菁醫學博士(左)主導,國內第一個完全由社會愛心挹注所完成的即時超音波檢查影像AI裝置,榮獲第33屆亞太肝臟研究醫學會研究者獎,臺大醫院影像醫學部主治醫師吳志宏醫學博士(右)代表報告與受獎。
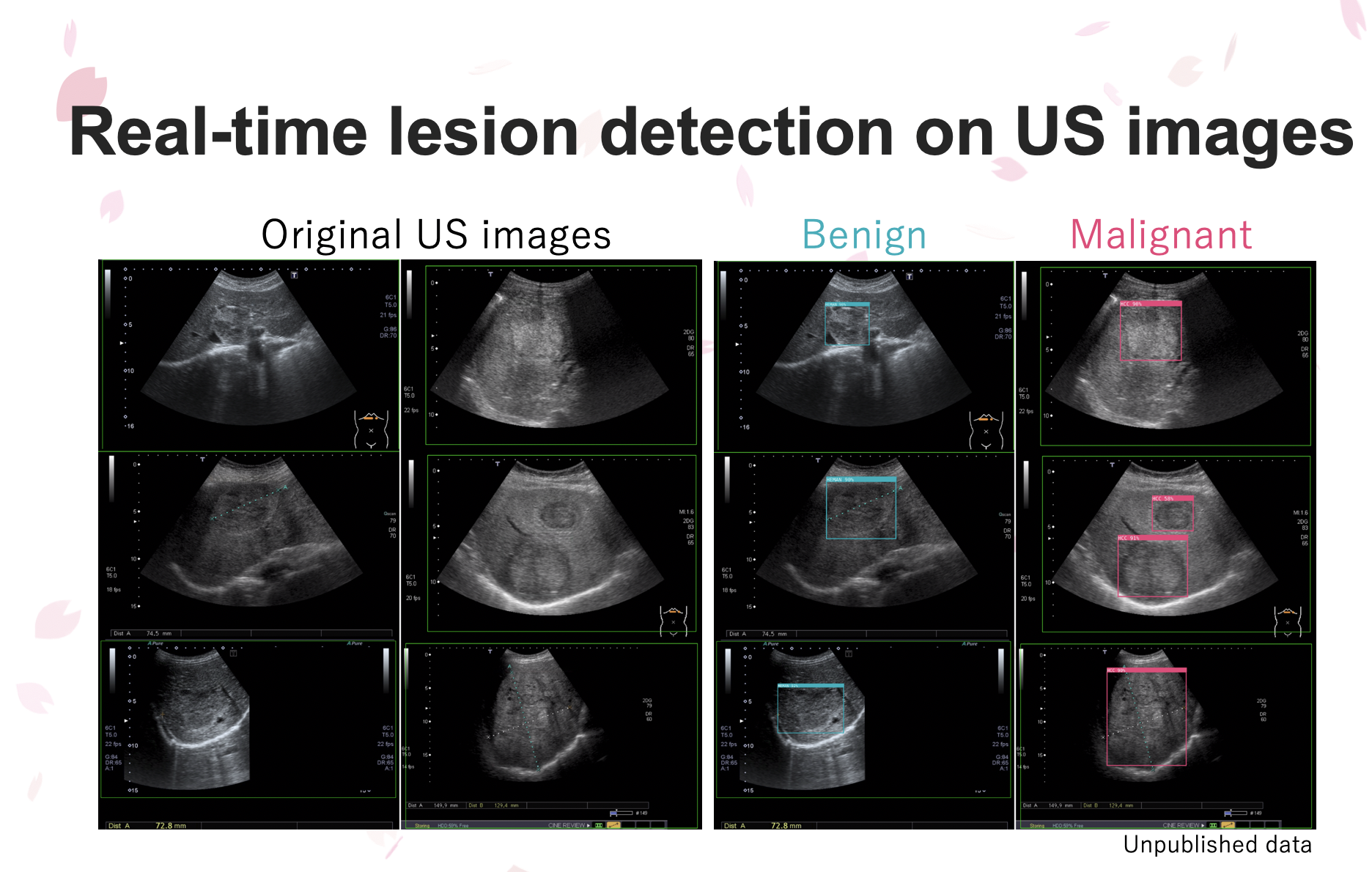
►:一般腹部超音波(圖左一及左二)只能靠醫療人員經驗找出可能的腫瘤病灶,但在AI即時超音波檢查影像AI裝置輔助下,肝腫瘤病變(圖右一紅框標出惡性腫瘤;圖右二藍框標出良性腫瘤)就能立即被偵測辨識,協助醫師即時診斷肝腫瘤良惡性。
The Asian Pacific Association for the Study of the Liver (APASL) 2024 Announces the Winner of the “Investigator Award”
Taiwan’s invention of an AI real-time detection and diagnosis of liver tumor with ultrasound won the award
Taiwan has made another world-wide medical advancement: the “deep learning device that automatically diagnoses and detects hepatic tumors,” developed by the Good Liver Foundation, the National Taiwan University Hospital, and Yuan High-Tech Development Co. Ltd., won the “Investigator Award” of the 33rd Asian Pacific Association for the Study of the Liver (APASL) held in Kyoto, Japan on March 30, 2024.
The device is the first entirely philanthropic real-time ultrasound AI in Taiwan. Yuan High-Tech Development Co. Ltd. supported the project monetarily and helped develop the device. Dr. Chih-Horng Wu, M.D., Ph.D., Attending of Department of Medical Imaging of the National Taiwan University Hospital, represented all involved parties to present the device and received the award at APASL, catching the attention of thousands of global liver medical experts.
The development of the device was headed by Dr. Hsiao-Ching Nien, M.D., Ph.D., the CEO of the Good Liver Foundation and the Attending of the Department of Family Medicine at the National Taiwan University Hospital. Dr. Nien hoped to replicate through AI the expertise and decades of experience of Professor Jin-Chuan Sheu, M.D., Ph.D. Pro. Sheu was the first expert to diagnose liver tumor with ultrasound in Taiwan. Therefore, when medical personnel use the device on patients for abdominal ultrasound, it utilizes Pro. Sheu’s expertise and longtime experience to differentiate whether the tumor is benign or malignant. Pro. Sheu is currently the Chairman of the Good Liver Foundation and the Honorary Professor of the National Taiwan University Hospital.
The invention collected data of 1,576 patients and 6,001 liver tumor images, and retrospectively confirmed diagnoses of liver tumors through computed tomography (CT), magnetic resonance imaging (MRI), or pathology. The images of tumors were marked by certified ultrasound imaging medical doctors, further training the AI device to automatically detect and diagnose liver tumors. Through these methods, the device can differentiate between benign and malignant tumor with 90% AUC(Area Under Curve), almost matching the diagnoses rates through CT or MRI.
Accordingly, the AI device could assist medical personnel to diagnose liver tumors using ultrasound in a timely manner and without radiation. It also relieves the anxiety of patients with liver tumors. Additionally, it reduces machine imaging (CT or MRI) waiting time and the Taiwan government’s medical expenses.
The device currently has received patents from many countries, with hopes that the device can soon be used clinically, benefiting patients, medial personnel, and countries with machine imaging inconvenience and expenditure difficulties.


















